The Truth About Lactate Threshold

By Taylor Byers, Swimming World College Intern.
Swimmers often encounter lactate sets throughout their training, yet several misconceptions exist about the body’s production of lactic acid and experiences of fatigue. Let’s start off with getting our definitions straight. The term “lactate threshold” is the maximum speed you can maintain without quickly tiring out. And while lactate is certainly associated with high levels of intense work (and can be measured easily with a blood sample), lactic acid simply does not exist within the body – our pH is too high. Lactate is actually a byproduct of fast energy breakdown that occurs during short, powerful bursts of work. It plays a positive role in the body and can indicate our level of fitness.

So if lactate and lactic acid don’t make us fatigued, then what causes the burning in your muscles while swimming fast? Here’s the true (and long) answer: the glycolysis process, which breaks down carbohydrates and other molecules to produce ATP. ATP is broken down during exercise, which produces energy and hydrogen molecules throughout the muscles. This buildup of hydrogen molecules is what causes the body’s pH to drop, making it more acidic and interfering with the muscle fiber’s ability to contract. When this occurs, it won’t be long before the swimmer has to shut down the intensity.
The glycolysis process produces pyruvate from the breakdown of carbohydrate, which subsequently bond with the hydrogen ions to create lactate in order to buffer the acid and help the body recover. During intense swimming, pyruvate cannot keep up with all of the hydrogen ions as they are being produced; thus, the excess hydrogen ions is what causes the burn and fatigue during swims. Lactate is often associated with the soreness encountered during intense workouts, so we will continue to refer to it as such.
Lactate threshold levels are determined by how well a swimmer’s body clears the hydrogen ions that are created. The faster the speed, the higher the rate of energy breakdown with subsequent higher levels of lactate. To find the maximum speed without overloading the body with acid, the goal is to improve the body’s ability to get rid of the lactate as quickly as possible once it starts to form. However, most people generate a lot more lactate than they are capable of clearing at high intensities.
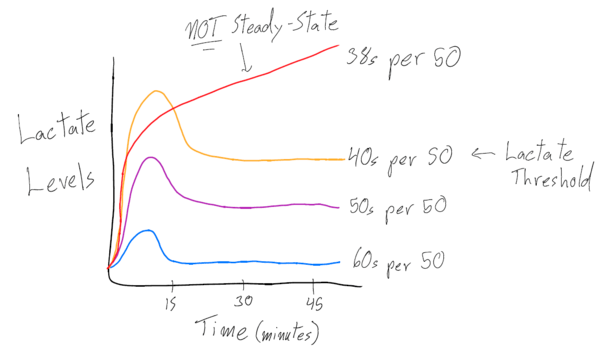
A way to measure the swimmer’s lactate levels is performing a lactate threshold set. If a swimmer starts off at a slower pace for 50s, lactate initially goes up for about 15 minutes or so, then decreases and is at a constant rate for a long time. If they are asked to swim at a faster pace, the lactate level spikes and then comes back down at a steady rate, but all at a higher level than before. Lastly, say the swimmer was asked to swim at an even faster pace than the first two times. The lactate shoots up high for the first few minutes, but then the swimmer starts to slow down and is unable to hold the pace anymore.
Looking at the diagram above, each line represents a specific intensity at which the swimmer completed the same distance, testing out which effort was at their lactate threshold. The red line depicts the athlete experiencing an exponential increase in lactate – the point at which the swimmer has reached their body’s threshold level of being able to clear lactate and metabolic waste product. Because the lactate levels did not come back down or level out from the initial spike, this swimmer found their threshold – the fastest pace speed they could maintain without the lactate levels skyrocketing.
Sprinters versus milers is a common comparison in lactate threshold. If a sprinter and a miler raced in a 100, the sprinter would win. This is because the race was not long enough for the lactate to build up and shut down the sprinter’s body. However, the sprinter’s lactate levels would be higher than a miler’s in a 100 race, because the sprinter is able to produce a lot of power from their glycolytic system, which results in a lot of lactate creation from the anaerobic emphasis. The miler is not able to produce a lot of power through the glycolytic system; thus, they struggle to produce high speed and a high level of lactate. The miler’s aerobic system is so efficient and programmed toward longer, less intense races, that it isn’t used to producing short bouts of speed and power.
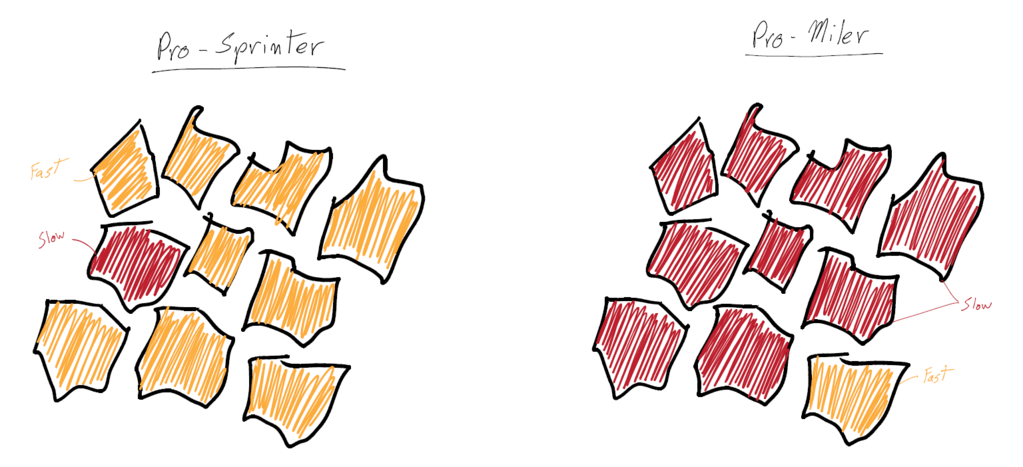
Many of the differences between a sprinter and a miler are genetic. Slow and fast twitch fibers greatly differ in lactate production. Milers have a lot of slow twitch fibers, which do not make a lot of acid (due to the aerobic nature of the muscle fibers), so they do not fatigue as easily. However, sprinters have a lot of fast twitch fibers, which produce massive amounts of power and lactate as a byproduct.

Another aspect that changes the peak lactate level is nutrition and recovery. By eating a low carb diet, the peak lactate level a swimmer can achieve will decrease by 25 percent. However, by eating a high carb diet, the peak lactate level a swimmer can achieve will increase 25 percent. This occurs largely due to the reliance on carbohydrate breakdown during short sprints through the glycolytic energy system.
All in all, the more swimmers practice doing lactate sets, the better they become. Raising your lactate threshold is a sign of becoming fitter and faster. Peak lactate levels can change, and the ability to produce higher levels can indicate improvement in performance. Since swimmers all over the world are pushing the peak higher and higher, the world of swimming just keeps getting faster and faster.
All commentaries are the opinion of the author and do not necessarily reflect the views of Swimming World Magazine nor its staff. All research was conducted by the author. Swimming and dryland training and instruction should be performed under the supervision of a qualified coach or instructor, and in circumstances that ensure the safety of participants.




THE MYTH OF LACTIC ACID…
There is considerable power behind the lactic acid myths in swimming, but the reality is that they are just that — myths. Let’s dispel them now.
Does lactic acid actually exist in swimmers?
According to Professor Matthew Hickey, head of the Human Performance Research Lab at Colorado State University, “The very bottom line is, NO. There is no lactic acid in swimmers.”
To explain why, a brief lesson about acid is necessary. Every acid has an alternate base form (alkaline) — a sort of yin and yang. For example, lactate is a base and lactic acid is, well, just that, an acid.
Acids and bases frequently exist as mixes and are able to change back and forth between one another.
So, how do we measure these acid/base mixes? Enter the “pH value,” which is simply a measure of acidity/alkalinity. The pH ranges on a scale from 0 to 14. So, the lower the number… the more acidic is our fluid. In this case a swimmers blood sample, for example which has a pH slightly above seven.
As the swimmers pH drops, more and more base molecules transform into acid molecules. There is a critical point at which there is a perfect 50/50 mix.
Professor Hickey points out that for lactic acid/lactate, that “occurs at a pH of 3.87.” That, of course, is well below our swimmers blood pH of seven.
And, this is why our swimmer never has “lactic acid” in his or her system. According to Professor Hickey, lactic acid really does not start appearing “until the swimmer has a pH of under six,” and even then “they would have 99 percent as lactate and one percent as lactic acid.
“During intense training, a swimmer can drive their pH levels down into the high sixes. This is well above the pH required to produce true lactic acid. If our swimmer had lactic acid in their blood, they would have to have a pH under six, and they would be rushed to hospital.” Hickey stated.
Why then do coaches and swimmers still talk about lactic acid in the body? Because of “simple historical inertia,” said Hickey. “It has stuck in the minds of swim coaches and swimmers, but it is based on a misunderstanding about the chemistry.”
This then begs the question if we do not have lactic acid, then what do we have? What causes the burn? Simply put… Our muscles do produce acid, but that acid is simply the positively charged hydrogen, not lactic acid as coaches and swimmer believe.
Scientists were long fooled because hydrogen and lactate exit the cell together — in fact one cannot leave without the other. So when we measure lactate levels, it correlates with hydrogen ions.
Coaches believed they were measuring lactic acid, but it is merely a coincidence that when we measure a rise in lactate it happens to match with a rise in the very painful hydrogen ion acid levels.
Does lactate serve a purpose?
Coaches and swimmers misunderstanding about lactate gave it a bad rap. In some quarters it is still believed that lactate is just a nasty waste product that made the swimmers legs and arms scream 15 meters from the finish of their race.
In reality, lactate serves many important roles. For example, “it is the principle fuel for the heart during vigorous exercise,” Hickey went on.
And swimmers liver can recycle it, thus, releasing a brand new glucose molecule… as if the swimmer had been drinking a sports drink.”
The reality is that as swimmers and coaches, we are constantly producing lactate in our bodies.
“There is also a misunderstanding that until you get to threshold you are not making lactate. That’s not the case,” Hickey said. “You are making lactate 24/7 all your life.”
The same goes for those hydrogen ions. The reason our blood lactate levels are low most of the time is because our bodies clear and use it as quickly as we produce it.
The swimmer’s lactate threshold is simply the point where the swimmer’s body produces both lactate and + Hydrogen ions faster than it can clear them.
This makes our swimmer’s ability to clear lactate and acid a critical part of sustaining high-end power
GO RACE ~ DARE TO SWIM FASTER
Paul Koster
Tay Thomas
Excellent topic about lactate.. iam very interested about all this kind of knowledge about lactate